| Category | Assignment | Subject | Education |
|---|---|---|---|
| University | Universiti Teknologi MARA (UiTM) | Module Title | CPE501 Chemical Process Control |
| Assessment Title | Group Work: Mini Project 1 |
|---|
This assignment utilizes the approach of problem-based learning related to process control. This assignment is motivated by understanding the fundamentals of chemical process control system design in a chemical process plant by analyzing the given process description.
This project addresses three (3) course outcomes and three (3) programme outcomes, which are mapped to complex engineering problem characteristics (WP) and the required knowledge profiles (WK) as shown in Table 1.
Table 1: Mapping of Course Outcomes (COS), Programme Outcomes (POS), Complex Engineering Problems (WPS) & Knowledge Profiles (WKS)
Hire Experts to solve PHA3005 Assignment before your Deadline
Pay & Buy Non Plagiarized AssignmentAt the end of this project, students should be able to:
A client has appointed your engineering consultancy firm to design a process flow that is complete with the design of the process control systems for the entire plant. The process description agreed between the management team of your firm and the client who is investing in the project is given hereafter.
A new hydrogen production plant with a production rate of 100 kilotonne per annum (kTa) needs to be designed based on methane pyrolysis technology. In the reaction part of the process, methane pyrolysis (Equation 1) is the only reaction that takes place in the reactor and all other possible reactions are assumed negligible. Therefore, the selectivity of hydrogen is 100%. Methane conversions of 70 mol% are achieved catalytically at 1050 °C in the bubble column reactor containing molten ABC catalyst. The feed gas contains pure methane without the presence of any impurities. The plant is designed based on 336 operating days per year.
CH. (gas) →2 H2 (gas) + C(solid)
(Equation 1)
The mixture of hydrogen product and unreacted methane in the reaction gas product stream is separated into a hydrogen-rich stream of 99 wt.% hydrogen purity and a methane-rich stream (>99 wt.% methane purity). The methane-rich stream is recycled back to join the fresh methane feed into the reactor. The detailed description of the process is given below.
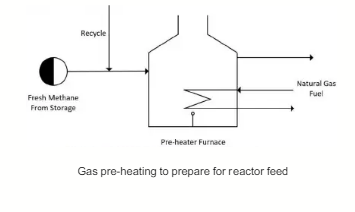
The fresh pure methane feed (Stream 1) flows from the storage tank at 13 bar and 30 °C is joined with the recycled unreacted methane stream coming from the membrane separator unit at the same pressure and 40 °C. The methane mixture (Stream 2) is preheated in a pre-heater furnace to 600 °C. The temperature is limited to below 650 °C to buffer the potential of thermal cracking. The preheat temperature is crucial because too low of a temperature causes the time taken to reach the final conversion to be longer. The pressure drop across the pre-heating furnace is 1 bar. The preheater furnace is run using natural gas fuel.
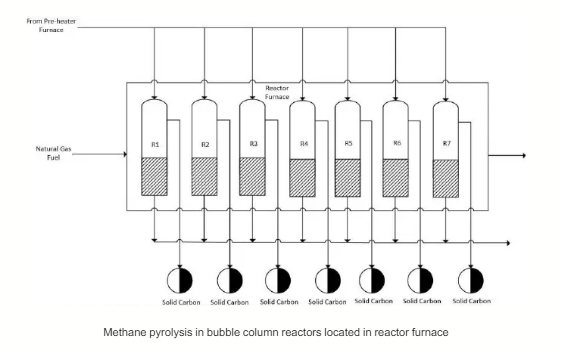
The pre-heated methane stream (Stream 3) is fed into 7 units of bubble column reactor tube arranged in parallel, placed in a single large furnace. The reactor furnace is run using natural gas fuel. This reactor design could be envisaged to resemble the steam methane reformer (SMR) where a bundle of multiple reactor tubes is placed in a single large furnace. At 1050 °C, the single pass conversion in each reactor tube is 70%. The overall conversion is also 70% since each reactor tube has equal flow of methane and equal amount of molten ABC catalyst (i.e., feed flowrate equally distributed into individual reactor tube containing equally distributed amount of catalyst). The pressure drop across each reactor tube is 3 bar. The design pressure of reactor tube material is 20 bar.
As shown in Equation 1, the methane pyrolysis reaction generates solid carbon as a byproduct. The solid carbons produced in each reactor accumulates and floats on the top surface of the molten ABC catalyst due to density difference and they are removed continuously in a separate stream from the product gas stream by an innovative mechanical work that takes place in the reactor. All the product streams coming out of each of the 7 reactor tubes are mixed in the pipeline and then flow in a single stream before entering the subsequent unit, which is the heat recovery steam generator (HRSG) system.
The process gas stream at the reactor exit containing hydrogen product and unconverted methane at 1050 *C must be cooled down before entering the membrane separator unit to separate the two gas components. The massive heat loss from the cooling process is utilized in a waste heat boiler (WHB) system, which is a heat recovery steam generator (HRSG) system to produce high pressure (HP) steam that can be utilized for electricity generation (co-gen plant). Due to the high flowrate of Stream 6, and hence, high amounts of waste heat to be recovered, the total duty is divided equally into 8 WHB units in the HRSG system.
The process gas stream coming out of the HRSG is assumed to be at the same pressure as its inlet, which is 9 bar. Water enters each WHB at 40 bar and 30 °C, and leaves as steam at 540 °C and the same pressure as its inlet. All the process gas streams leaving each of the 8 WHB units (Stream 7) at 30 °C are mixed in the pipeline and then flow in a single stream before entering the subsequent unit, which is the compressor.
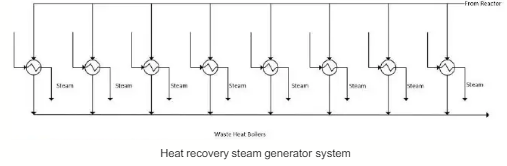
Heat recovery steam generator system
The pressure of the cooled product gas stream leaving the HRSG system is compressed from 9 bar to 14.5 bar.
The increase of stream pressure by the compressor causes the temperature of the process stream (Stream 8) to rise to 72.45 °C. Thus, a cooler is applied after the gas compression to cool down the process gas stream (Stream 9) to 40 °C with a pressure drop of 0.5 bar before entering the membrane separator. Cooling water is used to reach the desired temperature of the process gas.
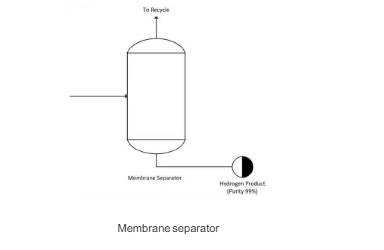
The gas mixture stream then undergoes a separation process in the membrane separator unit to separate the hydrogen product from the unreacted methane. Two streams leaving the membrane separator unit are a hydrogen-rich (99 wt.% purity) stream and a methane-rich stream (>99 wt.% purity). The methane-rich stream is then recycled back to join the fresh methane feed (Stream 1) in the pipeline prior to pre-heating in the pre-heater furnace. The temperature of the both the outlet streams leaving the membrane is assumed to be constant at its inlet stream temperature, while the pressure drop for the membrane separator is 1 bar. The separation efficiency of the membrane separator relies on the inlet flowrate to the membrane unit because the unit can only operate gas mixture flowrates that are in the range of ±10% of its nominal design flowrate. The design pressure of the membrane separator column material is 20 bar.
a.CO3/PO4/P5
Construct a process flow diagram (PFD) for the process using appropriate drawing software. (40 marks)
b. CO1/PO2/C4
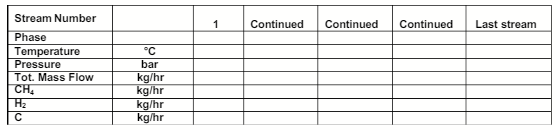
Develop the stream table for the process (fill in the cells in the table below for all streams). To develop the table, you will first need to compute the flowrate of fresh methane feed (in kg/hr) required for the process based on the information given in the process description. Subsequently, compute the flowrates of all the streams (in kg/hr) in the process. (20 marks)
C.CO1/PO2/C4
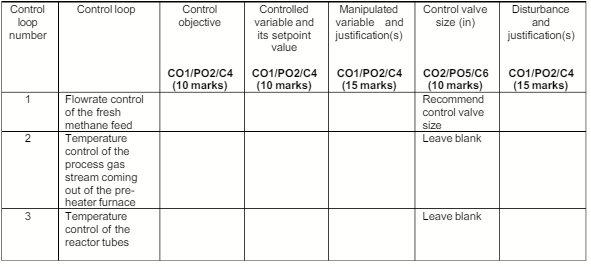
Analyze each of the identified feedback control loops below to determine the control objective, controlled variable and its setpoint value, manipulated variable with justification(s), and one disturbance. For the disturbance, relate how it affects the controlled variable.
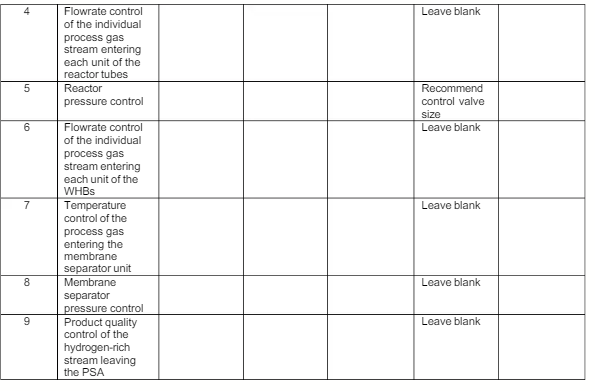
d. CO2/PO5/C6
Recommend the size of the control valve to be installed in each of the manipulated variable pipelines identified in the table below, using critical factor of 0.9, pressure drop across valve of 10 psi, and 100% overcapacity for all the control valves.
Present your analysis in table format as below:
e.CO3/PO4/P5
Construct a process and instrumentation diagram (P&ID) for the entire plant by modifying the PFD to include the control loops for all the control objectives identified above.
f. CO2/PO5/C6
In your P&ID, asses any crucial locations to install the pressure relief valves that serve as a safety measure to prevent explosions in the plant and justify your recommendations. (40 marks)
g. CO2/PO5/C6
Recommend the size of the control valve with 50% overcapacity for the cooling water service in the cooler prior to the membrane unit. The cooling water enters the cooler at 30 °C and leaves at 60 °C. The pressure drop across the valve is 10 psi. Assume constant specific heat capacity for both methane and hydrogen (Cpc = 2.232 kJ/kg°C, Cp2 = 14.3 kJ/kg°C). (10 marks)
Achieve Higher Grades PHA3005 Assignment & Raise Your Grades
Order Non Plagiarized Assignment
Are you looking for CPE501 Chemical Process Control Assignment? Worry no need! Ai–Free Chemical Engineering Assignment Help you score high in the academic year without any hassle. Our experts provide 100% human written and well-structured assignments. Our Best Assignment Help will make you productive and help you achieve high grades in your academic year. Hire cheap assignment helpers today and complete your assignment before the deadline. Free assignment samples are also provided to determine the quality and structure of the assignment. Hire professionals now! And stay worry-free!
Hire Assignment Helper Today!
Let's Book Your Work with Our Expert and Get High-Quality Content
