| Category | Assignment | Subject | Science |
|---|---|---|---|
| University | Nanyang Technological University | Module Title | CH2123 Chemical Thermodynamics |
By using Lee/Kesler generalized correlation below, calculate the fugacity of isobutane at 154 °C and 8620 kPa.
Generalized correlation defines the compressibility factor of a pure component as:
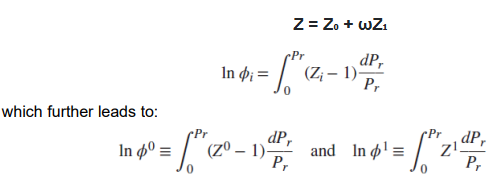
where Z₀ and Z₁ are functions of (Pr, Tr), which are the reduced pressure and reduced temperature, respectively, and ω is the accentricity factor of the pure component.
The equation for fugacity coefficient φi can now be written in terms of reduced pressure, which further leads to:
(Refer to Appendix D Tables D.13-D16, Smith Van Ness, Introduction to Chemical Engineering Thermodynamics 8th Ed., 2018)
The vapor-liquid equilibrium data for a binary liquid solution containing acetone (1) – methanol (2) at 55 °C is given in Table 1. Assume that the vapor phase can be considered as ideal gas mixture whereas the activity coefficients for acetone (1) and methanol (2) can be expressed by Van Laar model as follows:
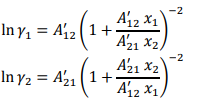
Determine the parameters A’₁₂ and A’₂₁ (both of which are constants) that best fit the data in Table 1.
By using Excel, construct P-x, y diagram at 55 °C based on the suggested model above, and compare it with the experimental data (see Table 1).
Compare the azeotropic composition based on the experiment and the model above.
Note: The saturated vapor pressures of each component can be determined by using Antoine equations from any textbook/other reference.
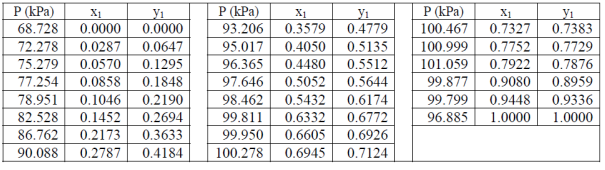
Table 1. VLE data for acetone (1) – methanol (2) mixture at 55 °C.
Submit Your Assignment Questions & Get Plagiarism Free Answers.
Order Non Plagiarized AssignmentAqueous solution of perfluorotributylamine can be considered as artificial blood because of its high oxygen solubility.
At 25 °C and an oxygen pressure of 1 atm, 380 mL of oxygen gas (measured at 25 °C and 1 atm) dissolves in 1 liter of pure perfluorotributylamine, which has a liquid density of 1.88 g/mL.
Determine the Henry’s Law constant, in unit of atm, for oxygen dissolved in pure perfluorotributylamine.
Suppose a solution contains 10 vol% perfluorotributylamine and 90 vol% water. Estimate the volume of oxygen gas (measured at 25 °C and 1 atm) dissolved in a liter of the solution when it is equilibrated with air at 25 °C. Note that the Henry’s constant for oxygen in water at 25 °C is 4.34 × 10⁴ atm.
Note: The physical properties of perfluorotributylamine can be obtained from external reference.
Stuck on your CH2123 Chemical Thermodynamics? Don't worry! Our Science Assignment Help service is the best for you. If you need help with assignments, our expert PhD writers will provide you with original content. And yes, you will also get free assignment samples, which will give you a perfect idea of how to write a top-quality assignment. Don't worry about the deadline, as we guarantee on-time delivery. Contact us now for high-quality and plagiarism-free work and boost your grades!
Hire Assignment Helper Today!
Let's Book Your Work with Our Expert and Get High-Quality Content
